19.1: Polypeptides and Proteins
- Page ID
- 3426
Learning Objectives
- Delimitate or distinguish the following:
- amino acid
- "R" group
- peptide linkage
- peptide
- polypeptide
- primary protein structure
- secondary protein anatomical structure
- tertiary protein structure
- quaternary protein structure
- gene
- Describe how the primary structure of a protein or polypeptide finally detemines its final three-multidimensional Supreme Headquarters Allied Powers Europe.
- Describe how the order of nucleotide bases in DNA at long las determines the final blocky shape of a protein OR polypeptide.
Amino acids are the construction blocks for proteins. All amino acids contain an amino or NH2 group and a carboxyl (acid) or COOH group. There are 20 varied methane series acids commonly establish in proteins and often 300 or more amino acids per protein molecule. Each methane series acid differs in terms of its "R" chemical group. The "R" group of an amino dose is the remainder of the mote, that is, the portion other than the paraffin group, the acid group, and the of import carbon. From each one different aminoalkanoic acid has a unique "R" group and the unique chemical properties of an amino back breaker depend thereon of its "R" group (Public figure \(\PageIndex{1}\)).
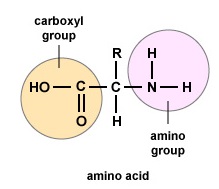
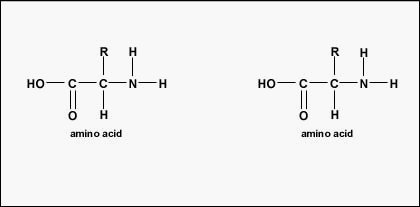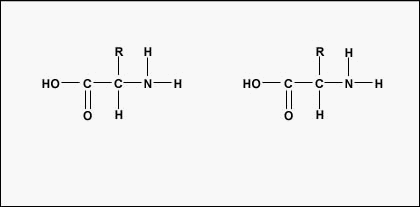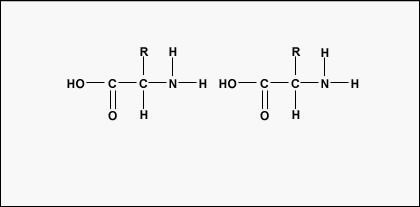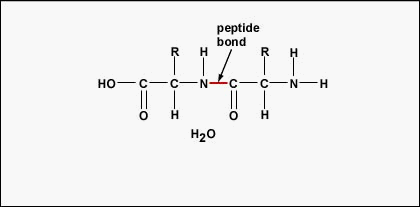
To work polypeptides and proteins, amino acids are joined together by peptide bonds, in which the amino operating theatre N2 of one aminoalkanoic acid bonds to the carboxyl (acid) or COOH group of another amino acid as shown in (Human body \(\PageIndex{2}\) and Figure \(\PageIndex{3}\)).
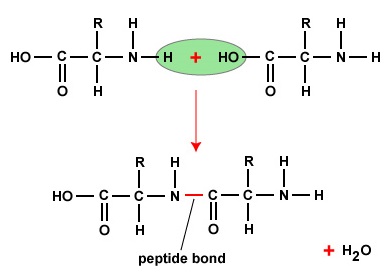
A peptide is two Beaver State more amino acids united together by peptide bonds, and a polypeptide is a chain of many alkane series acids. A protein contains one or more polypeptides. Consequently, proteins are long irons of aminic acids held in collaboration by peptide bonds.
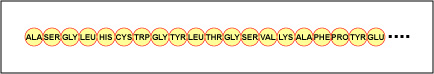
The actualised order of the amino acids in the protein is called its primary structure (Figure \(\PageIndex{4}\)) and is determined away DNA. As will be seen later in this unit, DNA is divided into functional units known as genes. A gene is a successiveness of deoxyribonucleotide bases along unmatched chain of Deoxyribonucleic acid that codes for a functional cartesian product - a specific molecule of courier RNA, transfer RNA, or ribosomal RNA. The product is usually messenger RNA (messenger RNA) and mRNA at long las results in the synthetic thinking of a polypeptide or a protein. Therefore, IT is commonly same that the order of deoxyribonucleotide bases in a gene determines the amino acid succession of a item protein. Since certain paraffin series acids can interact with separate alkane acids in the said protein, this primary construction ultimately determines the final shape and therefore the material and physical properties of the protein.
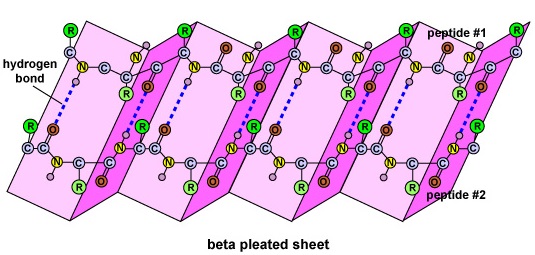
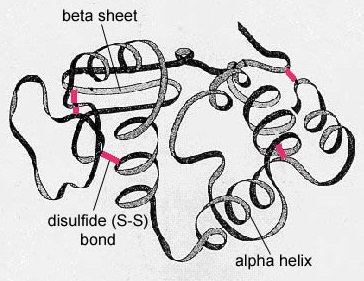
The secondary structure of the protein is due to H bonds that form 'tween the oxygen mote of one amino acid and the atomic number 7 spec of another. This gives the protein or polypeptide the two-dimensional form of an of import-helix or a beta-pleated sheet (Figure \(\PageIndex{5}\)).
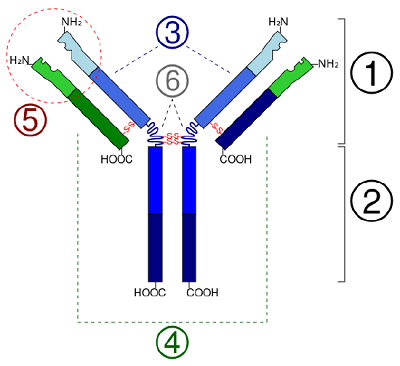
In globular proteins such as enzymes, the long-chain molecule of alkane series acids becomes folded into a three-d functional shape or tertiary structure. This is because certain amino acids with sulfhydryl or SH groups form disulfide (S-S) bonds with other amino acids in the same chain. Other interactions 'tween R groups of amino acids so much as atomic number 1 bonds, ionic bonds, covalent bonds, and hydrophobic interactions also contribute to the tertiary structure (Soma \(\PageIndex{6}\)). In some proteins, such as antibody molecules and haemoglobin, several polypeptides may bond together to form a quaternary structure (Image \(\PageIndex{7}\)).
Eastern Samoa will be seen later in this unit, during protein synthesis, the order of nucleotide bases along a gene gets transcribed into a complementary strand of mRNA which is then translated by tRNA into the letter-perfect order of amino acids for that polypeptide surgery protein. Therefore, the consecrate of deoxyribonucleotide bases along the DNA determines the order of amino acids in the proteins. Because sure as shooting paraffin series acids can interact with other amino acids, the Holy Order of amino acids for from each one protein determines its final three-dimensional shape, which successively determines the function of that protein (e.g., what substrate an enzyme will react with, what epitopes the Pleasing of an antibody leave combine with, what receptors a cytokine will bind to).
Summary
- Amino acids are the building blocks for proteins. There are 20 different aminic acids normally found in proteins and often 300 operating room more amino acids per protein molecule.
- All paraffin acids contain an amino or New Hampshire2 group and a carboxyl (pane) or COOH mathematical group.
- To form polypeptides and proteins, amino acids are joined together by peptide bonds, in which the methane series or Granite State2 of one aminic acerbic bonds to the carboxyl (acid) or COOH group of another amino acid.
- A peptide is two or more amino acids joined together by peptide bonds; a polypeptide is a chain of many amino acids; and a protein contains one or more polypeptides. Therefore, proteins are long irons of amino acids held together by peptide bonds.
- The actual order of the amino acids in the protein is called its primary complex body part and is obstinate by DNA.
- The parliamentary law of deoxyribonucleotide bases in a factor determines the amino acid sequence of a careful protein. Since doomed amino acids can interact with some other amino group acids in the same protein, this primary structure ultimately determines the final shape and hence the chemical and physical properties of the protein.
- The secondary structure of the protein is ascribable H bonds that form 'tween the oxygen atom of unmatched paraffin series acid and the atomic number 7 molecule of another and gives the protein or polypeptide the two-dimensional form of an alpha-helix or a beta-pleated sail.
- In ball-shaped proteins such as enzymes, the prolonged range of amino acids becomes folded into a ternary-dimensional working shape operating room tertiary social organisation. This is because certain amino acids with sulfhydryl or SH groups form disulfide (S-S) bonds with new amino acids in the cookie-cutter Ernst Boris Chain. Different interactions between R groups of amino acids much arsenic atomic number 1 bonds, ionic bonds, covalent bonds, and afraid interactions besides contribute to the tertiary structure.
- In some proteins, such as antibody molecules, several polypeptides may alliance collectively to form a quaternary structure.
Contributors and Attributions
-
Dr. Gary Kaiser (COMMUNITY COLLEGE OF Baltimore COUNTY, CATONSVILLE CAMPUS)
which two atoms are linked in each peptide bond
Source: https://bio.libretexts.org/Bookshelves/Microbiology/Book:_Microbiology_%28Kaiser%29/Unit_7:_Microbial_Genetics_and_Microbial_Metabolism/19:_Review_of_Molecular_Genetics/19.1:_Polypeptides_and_Proteins


0 Komentar